When drawing electron dot or Lewis Diagrams, always keep in mind that the nucleus is represented by the atomic symbol.
Dots represent electrons in electron dot diagrams.
Lines represent the bond of two electrons in Lewis Diagrams - also known as structural diagrams.
There are four orbitals - at the north, east, south, and west sides of the nucleus as shown.
Now in a covalent compound:
While hydrogen has one valence electron, chlorine has 7. When they are drawn like this, it means that hydrogen and chlorine are sharing the two electrons between them. These two electrons can also be represented as a horizontal line in a structural diagram.
Now in ionic compounds:
In the first example, Na has lost an electron and is now an ion with a +1 charge - it now has an empty valence shell and is stable. Cl has gained this electron and is now an ion with a -1 charge - it now has a full valence shell and is stable. These two ions are held together by their opposite attraction.
Diagrams for Polyatomic Ions
Because polyatomic ions have charges - electrons must be added or taken away. I find that it is easiest to do this after we have paired up the existing electrons. In the above example, we see CO3. After pairing up carbon's four valence electrons with those of the two oxygens on the left and on the right, we see that we need two more electrons in order to pair with the third oxygen on top. The 2- subscript means that the two electrons have been added.
Enjoy!


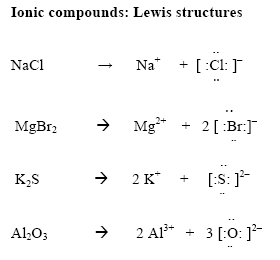

No comments:
Post a Comment