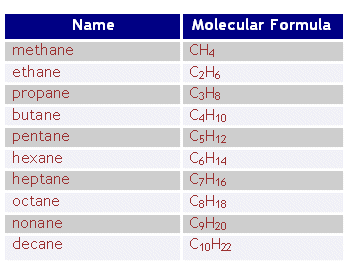
The naming rule is exactly the same thing as Alkanes. Positioning bonds is based if the bonds have the lowest number and if it is put in front of the parent chain.
Alkenes are made up with only hydrogen and carbon (hydrocarbon). Hydrocarbon with on or more double bonds are placed between carbon atoms. The ending changes from ~ane to ~ene.
Try this!
This is a 4-methyl 2-pentene
ex. Try to draw 2,5-dimethyl-2-heptene
CH3 CH3 | | CH3CH2CHCH2CH=CCH3
Geometric Isomers are only used in Alkenes. They have the same formula but have different arrangements.
Trans and Cis
 Use the example of Butane, this is a Cis because the H is on the same side as each other and CH3 or H3C is on the same side as each other.
Use the example of Butane, this is a Cis because the H is on the same side as each other and CH3 or H3C is on the same side as each other. This is another example of Butane but this is a Trans because H and CH3 are diagonal from each other and are not on the same side.
This is another example of Butane but this is a Trans because H and CH3 are diagonal from each other and are not on the same side. Alkynes are hydrocarbons that make triple bonds and it to is positioned between between carbon atoms. When naming the ending changes from ~ane and ~ene to ~yne
Try practicing some on this website! Practice Naming
Jokes of the day!! HAHAHAHAAAA
A group of organic molecules were having a party, when
a group of robbers broke into the room and stole all
of the guests joules.A tall, strong man, armed with a
machine gun came into the room and killed the robbers
one by one.The guests were very grateful to this man,
and they wanted to know who he was. He replied: My
name is BOND, Covalent Bond.
Jokes of the day!! HAHAHAHAAAA
A group of organic molecules were having a party, when
a group of robbers broke into the room and stole all
of the guests joules.A tall, strong man, armed with a
machine gun came into the room and killed the robbers
one by one.The guests were very grateful to this man,
and they wanted to know who he was. He replied: My
name is BOND, Covalent Bond.