No measurement is exact; each measurement is only an estimate which means that there is some sort of uncertainty involved. An exception to this is when you are able to physically count the objects. Counting people would be a good example of that.
Uncertainty:
There are two types of uncertainties; Absolute Uncertainty and Relative Uncertainty.
Absolute Uncertainty:
-expressed in units of measurement and not in ratios
First Method of Absolute Uncertainty:
1) Cross out unreasonable data
2) Calculate the average of the other measurements
3) The absolute uncertainty is the largest difference between the average plus/minus the lowest or highest reasonable measurement
Example: The following measurements are in cm:
20.0, 20.2, 20.3, 20.5, 23.0
First of all, you want to cancel out the 23.0 because it has a big difference from the other measurements.
Then, you calculate the average from the rest of the numbers which turns out to be 20.25
Now the measurement that will give you the biggest difference will be 20.5 so 20.5-20.25 = 0.25
The answer is 20.25 ± 0.25
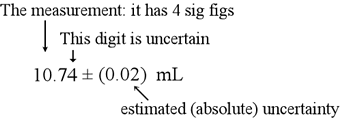 |
| For example, since '4' is the uncertain digit and is in the second decimal place, the uncertainty will be in the second decimal place as well. |
1) Determine how many increments the instrument go up by
2) Determine one tenth of that measurement
Relative Uncertainty:
-Relative Uncertainty can be expressed as:
a) percentage (%)
b) by using significant figures
Absolute Uncertainty
Relative Uncertainty = --------------------------------------
Estimated Measurement