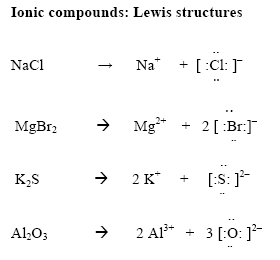Functional groups- specific group of atoms in a molecule & gives the molecule the ability to react in a specific manner/give it special properties.
They're organic compounds that contain elements other than Carbon and Hydrogen. It can include single atoms (Ex. Cl, Br) or groups of atoms (Ex. NO2, NH2)
Examples include alcohol, aldehydes, amines, ethers, ester, etc.
The main ones we will focus on are:
1) Halides & Nitro Compounds
2) Alcohols
3) Aldehydes & Ketones
Halides & Nitro Compounds:
It's named similar to hydrocarbons and are attached to alkenes, alkanes, and alkynes.
How to name them? Main chain names receieve the following prefixes if that certain group is attached:
Halogens:
F=fluoro
Cl= chloro
Br= bromo
I= iodo
Nitro:
NO2= nitro
Use a number to indicate where it's attached to the hydrocarbon chain. If more than one is present, use prefixes di, tri, tetra, etc. If it contains more than one group, put them in alphabetical order. Number from the end which gives the lowest set of numbers.
Properties of Halogenated Compounds:
1) Tend to be insoluble in water (similar to alkanes)
2) Fluorinated compounds tend to be unreactive (inert)
Ex. Teflon is resistant to reactions with most chemicals
3) Chloro and bromo compounds are susceptible to chemical attacks under drastic conditions
4) Iodo compounds are more reactive and can be easily replaced by other functional groups
Properties of Nitro Compounds:
1) Soluble in water
2) Unreactive to chemical attacks except under drastic conditions
3) Explosive (Ex. TNT (trinitrotulene) and nitroglycerine)
4) Pleasant odour.
Alcohols- organic compounds containing OH group
How to name it? Number the hydrocarbon chain and give the lowest possible number for the OH group. Put the number before the name of the parent hydrocarbon, separated by a dash. Alkyl groups are placed in front of number for the OH. Change the 'e' ending to 'ol'. An example of an alcohol is in the picture on the left which is ethanol. And oh-so happens to look like a dog. LOL.
Properties of Alcohols:
1) OH group tends to make alcohols soluble in water. Non polar hydrocarbons chains make it insoluble.
Methanol, ethanol, and propanol are highly soluble in water due to hydrocarbon being small. Butanol is moderately soluble. Penntanol and high are insoluble in water.
2) Poisonous to some degree. Includes ethanol (in alcoholic beverages)
Multiple-OH:
If a compound has more than one group, number both and add diol, triol, etc endings.
Aldehydes- organic compound containing a C=O group at the end of the hydrocarbon chain
It follows the standard rules but changes the parent chain ending to 'al'. The simplest of this group is methanal (formaldehyde).
Ketones-organic compound containing C=O group at a position other than the end of the hydrocarbon chain.
It follows the standard rules but add 'one' at the ending to the parent chain.
Thursday, June 2, 2011
Tuesday, May 31, 2011
Alkenes and Alkynes
So what's so special about Alkenes and Alkynes? Well the big secret is that they can form DOUBLE and TRIPLE bonds! (carbon only).
 Use the example of Butane, this is a Cis because the H is on the same side as each other and CH3 or H3C is on the same side as each other.
Use the example of Butane, this is a Cis because the H is on the same side as each other and CH3 or H3C is on the same side as each other. This is another example of Butane but this is a Trans because H and CH3 are diagonal from each other and are not on the same side.
This is another example of Butane but this is a Trans because H and CH3 are diagonal from each other and are not on the same side.
The naming rule is exactly the same thing as Alkanes. Positioning bonds is based if the bonds have the lowest number and if it is put in front of the parent chain.
Alkenes are made up with only hydrogen and carbon (hydrocarbon). Hydrocarbon with on or more double bonds are placed between carbon atoms. The ending changes from ~ane to ~ene.
Try this!
This is a 4-methyl 2-pentene
ex. Try to draw 2,5-dimethyl-2-heptene
CH3 CH3 | | CH3CH2CHCH2CH=CCH3
Geometric Isomers are only used in Alkenes. They have the same formula but have different arrangements.
Trans and Cis
 Use the example of Butane, this is a Cis because the H is on the same side as each other and CH3 or H3C is on the same side as each other.
Use the example of Butane, this is a Cis because the H is on the same side as each other and CH3 or H3C is on the same side as each other. This is another example of Butane but this is a Trans because H and CH3 are diagonal from each other and are not on the same side.
This is another example of Butane but this is a Trans because H and CH3 are diagonal from each other and are not on the same side. Alkynes are hydrocarbons that make triple bonds and it to is positioned between between carbon atoms. When naming the ending changes from ~ane and ~ene to ~yne
Try practicing some on this website! Practice Naming
Jokes of the day!! HAHAHAHAAAA
A group of organic molecules were having a party, when
a group of robbers broke into the room and stole all
of the guests joules.A tall, strong man, armed with a
machine gun came into the room and killed the robbers
one by one.The guests were very grateful to this man,
and they wanted to know who he was. He replied: My
name is BOND, Covalent Bond.
Jokes of the day!! HAHAHAHAAAA
A group of organic molecules were having a party, when
a group of robbers broke into the room and stole all
of the guests joules.A tall, strong man, armed with a
machine gun came into the room and killed the robbers
one by one.The guests were very grateful to this man,
and they wanted to know who he was. He replied: My
name is BOND, Covalent Bond.
Sunday, May 29, 2011
Organic Chemistry.
Organic Chemistry is also known as the chemistry of carbon compounds.
Organic Compounds have low melting points, and can form chains of carbon atoms, linked in a straight-line, circular pattern, or branched pattern.
They can also link with other atoms in either a single bond, double bond or triple bond.
A hydrocarbon is a compound comprised of only hyrogen and carbon.
Alkanes are saturated hydrocarbon, in which the carbon atoms are bonded with single bonds.
Names of Alkanes: Here is a table of the differet alkanes and its molecular formula.
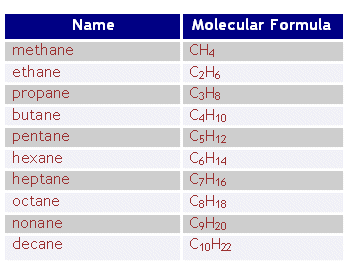

The formula for writing the formula of alkanes is: CnH2n+2
Branched hydrocarbons or Substituted hydrocarbons are hydrocarbon attached to the side of the original hydrocarbon.

A alkyl group is an aklane which has lost a hydrogen atom.
Thursday, May 19, 2011
Chemical Bonding!
3 Types of Bonding:
1) Ionic bonding- the transfer between 2 atoms to form a positive ion and negative ion.
2) Non-polar covalent bonding- equal sharing of electrons
3) Polar covalent bonding- unequal sharing of electrons
So let's start with ionic bonding. This uses the electrostatic force. This is the force that exists between charged particles as a result of attraction/repulsion. It operates equally in all directions. It's important to know that ionic bonds are very strong, so compounds held together with this bond have a high melting temperature.
We know that in an ionic bond, a metal and non metal combines. Why does the metal lose valence electrons? This is explained by electronegativity. Metals have a low electronegativity and non metals have high electronegativity which results in high ionization energies. The difference in this electronegativity will determine the electron sharing. The Pauling Scale (picture) measures electronegativity. To find the electronegativity difference, you can use this formula:
Now about non polar covalent bonding. It involves equal sharing to satisfy the octet rule. It is VERY STRONG. Now for some of these bonds such as CH4, O2, and F2, they have low melting points. Why? Because of weak bonds.
So what holds the bonds together? Intramolecular forces that are found within the molecule, and responsible to hold the atoms of molecules together. Intermolecular forces are between molecules and are responsible for the bonding between molecules.
London Forces are weak attractive forces due to temporary dipolar attractions between neighbouring atoms. They can exist individually or parts of a molecule. Also the greater the atomic number of the atom, the stronger the London force occurs. What's a dipole? It's a partial separation of charge where one end of a molecule/bond has a slight excess positive charge and the other end has a slight excess of negative charge.
The last bonding is polarity which involves the molecule's electrical balance. If there's an imbalance, it's polar. If it's balanced, then it's non polar.
Electronegativity effects this because the greater of this it has, the greater pull of the electrons in the bond will be pulled more towards itself and the shared electrons spend more time near the atom. High electronegativity results in partial negative. (δ- between 0 & -1) Low electronegativity results in partial positive. (δ+ between 0 & +1) The δ symbol represents partial.
An arrow indicates the migration of electrons. It will point to the partial negative atom involved in the bond. An example is H2O:
H: 2.20
O:3.44
3.44-2.20= 1.24
This means it is a polar covalent bond.
1) Ionic bonding- the transfer between 2 atoms to form a positive ion and negative ion.
2) Non-polar covalent bonding- equal sharing of electrons
3) Polar covalent bonding- unequal sharing of electrons
So let's start with ionic bonding. This uses the electrostatic force. This is the force that exists between charged particles as a result of attraction/repulsion. It operates equally in all directions. It's important to know that ionic bonds are very strong, so compounds held together with this bond have a high melting temperature.
We know that in an ionic bond, a metal and non metal combines. Why does the metal lose valence electrons? This is explained by electronegativity. Metals have a low electronegativity and non metals have high electronegativity which results in high ionization energies. The difference in this electronegativity will determine the electron sharing. The Pauling Scale (picture) measures electronegativity. To find the electronegativity difference, you can use this formula:
ENeg Diff. = lENeg1 - ENeg 2l
If ENeg Diff <0.5 it's a non polar covalent bond
If ENeg Diff > 0.5 and <1.8 it's a polar covalent bond
If ENeg Diff > 1.8 it's an ionic bond
Now about non polar covalent bonding. It involves equal sharing to satisfy the octet rule. It is VERY STRONG. Now for some of these bonds such as CH4, O2, and F2, they have low melting points. Why? Because of weak bonds.
So what holds the bonds together? Intramolecular forces that are found within the molecule, and responsible to hold the atoms of molecules together. Intermolecular forces are between molecules and are responsible for the bonding between molecules.
London Forces are weak attractive forces due to temporary dipolar attractions between neighbouring atoms. They can exist individually or parts of a molecule. Also the greater the atomic number of the atom, the stronger the London force occurs. What's a dipole? It's a partial separation of charge where one end of a molecule/bond has a slight excess positive charge and the other end has a slight excess of negative charge.
The last bonding is polarity which involves the molecule's electrical balance. If there's an imbalance, it's polar. If it's balanced, then it's non polar.
Electronegativity effects this because the greater of this it has, the greater pull of the electrons in the bond will be pulled more towards itself and the shared electrons spend more time near the atom. High electronegativity results in partial negative. (δ- between 0 & -1) Low electronegativity results in partial positive. (δ+ between 0 & +1) The δ symbol represents partial.
An arrow indicates the migration of electrons. It will point to the partial negative atom involved in the bond. An example is H2O:
H: 2.20
O:3.44
3.44-2.20= 1.24
This means it is a polar covalent bond.
Tuesday, May 10, 2011
Drawing Electron Dot and Lewis Diagrams
When drawing electron dot or Lewis Diagrams, always keep in mind that the nucleus is represented by the atomic symbol.
Dots represent electrons in electron dot diagrams.
Lines represent the bond of two electrons in Lewis Diagrams - also known as structural diagrams.
There are four orbitals - at the north, east, south, and west sides of the nucleus as shown.
Now in a covalent compound:
While hydrogen has one valence electron, chlorine has 7. When they are drawn like this, it means that hydrogen and chlorine are sharing the two electrons between them. These two electrons can also be represented as a horizontal line in a structural diagram.
Now in ionic compounds:
In the first example, Na has lost an electron and is now an ion with a +1 charge - it now has an empty valence shell and is stable. Cl has gained this electron and is now an ion with a -1 charge - it now has a full valence shell and is stable. These two ions are held together by their opposite attraction.
Diagrams for Polyatomic Ions
Because polyatomic ions have charges - electrons must be added or taken away. I find that it is easiest to do this after we have paired up the existing electrons. In the above example, we see CO3. After pairing up carbon's four valence electrons with those of the two oxygens on the left and on the right, we see that we need two more electrons in order to pair with the third oxygen on top. The 2- subscript means that the two electrons have been added.
Enjoy!
Dots represent electrons in electron dot diagrams.
Lines represent the bond of two electrons in Lewis Diagrams - also known as structural diagrams.
There are four orbitals - at the north, east, south, and west sides of the nucleus as shown.
Now in a covalent compound:
While hydrogen has one valence electron, chlorine has 7. When they are drawn like this, it means that hydrogen and chlorine are sharing the two electrons between them. These two electrons can also be represented as a horizontal line in a structural diagram.
Now in ionic compounds:
In the first example, Na has lost an electron and is now an ion with a +1 charge - it now has an empty valence shell and is stable. Cl has gained this electron and is now an ion with a -1 charge - it now has a full valence shell and is stable. These two ions are held together by their opposite attraction.
Diagrams for Polyatomic Ions
Because polyatomic ions have charges - electrons must be added or taken away. I find that it is easiest to do this after we have paired up the existing electrons. In the above example, we see CO3. After pairing up carbon's four valence electrons with those of the two oxygens on the left and on the right, we see that we need two more electrons in order to pair with the third oxygen on top. The 2- subscript means that the two electrons have been added.
Enjoy!
Tuesday, May 3, 2011
Periodic Trends
So what are periodic trends?
They are progress of increasing or decreasing with the charactierstics of certain elements.
They're are a lot of trends and we must know them all:
1)Metallic Properties
2)Atomic Radius
3)Ionization Energy
4)Electronegativity
5) Reactivity
6)Ion Cahrge
7) Melting and Boiling Point
8) Density
Here are some of the trends...let's start of with...
Metallic Properties

The elements change from left to right arosss the table and elelements that are more metallic moves down the table.
Atomic Radius:

The atom decreases aross a row from left to right and increases going down a family. When the atom is going from left to right the atomic number increases along with the nucleus.
When the atoms are going down a family the orbits are more compact and therefore the inner electons attract each other whereas the elctrons on th outer end repel each other.
Reactivity:

Metals and Non-metals have and show different trends. When ther metal moves down a family and right a row it is more reactive and when going up and left it is less reactive.
Non-metals are the opposite or metals, it increases or it's more reactive as an atom goes up a family.
Melting and Boiling Point:

The elements from the center has the highest melting point and the Noble Gases has the lowest melting point.
Ionization Energy:

Ionization Energy are enegies needed to remove one elctron from an atom. They increase as it does up and right in t he periodic table and all the noble gases have high ionization energy. The elctrons have these atoms remove eaily. When going left to right it has a higher attraction between the nucleus and the elctrons whereas the outer electrons decreases as it goes down a group.
Electronegativity:

Electornegativity is how much atom wants to gain electrons. When the atoms goes left to right and up they increase and decreases going down a group Atoms with high electornegativity are strognly attracts other elctrons from another atom or it may remove an electron from a neighbor.
They are progress of increasing or decreasing with the charactierstics of certain elements.
They're are a lot of trends and we must know them all:
1)Metallic Properties
2)Atomic Radius
3)Ionization Energy
4)Electronegativity
5) Reactivity
6)Ion Cahrge
7) Melting and Boiling Point
8) Density
Here are some of the trends...let's start of with...
Metallic Properties
The elements change from left to right arosss the table and elelements that are more metallic moves down the table.
Atomic Radius:
The atom decreases aross a row from left to right and increases going down a family. When the atom is going from left to right the atomic number increases along with the nucleus.
When the atoms are going down a family the orbits are more compact and therefore the inner electons attract each other whereas the elctrons on th outer end repel each other.
Reactivity:
Metals and Non-metals have and show different trends. When ther metal moves down a family and right a row it is more reactive and when going up and left it is less reactive.
Non-metals are the opposite or metals, it increases or it's more reactive as an atom goes up a family.
Melting and Boiling Point:
The elements from the center has the highest melting point and the Noble Gases has the lowest melting point.
Ionization Energy:
Ionization Energy are enegies needed to remove one elctron from an atom. They increase as it does up and right in t he periodic table and all the noble gases have high ionization energy. The elctrons have these atoms remove eaily. When going left to right it has a higher attraction between the nucleus and the elctrons whereas the outer electrons decreases as it goes down a group.
Electronegativity:
Electornegativity is how much atom wants to gain electrons. When the atoms goes left to right and up they increase and decreases going down a group Atoms with high electornegativity are strognly attracts other elctrons from another atom or it may remove an electron from a neighbor.
Sunday, May 1, 2011
Periodic Trends
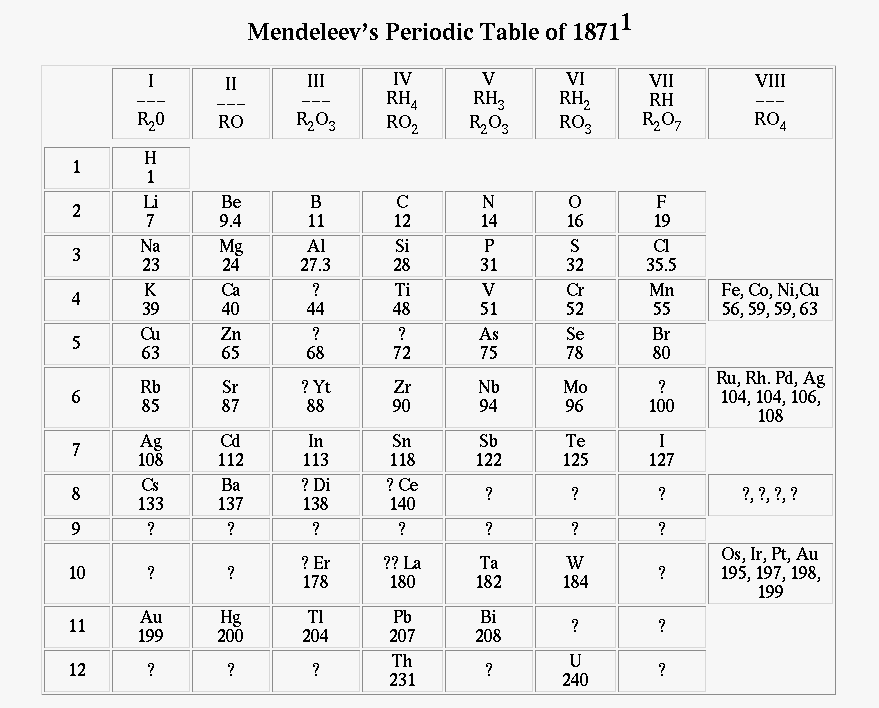
In 1870, Dmitri Mendeleev was the first person to decide to put all the elements on a "table" so that it could be more organized and easier to study. Since then, it has the periodic table has been made better along with additional elements.
To find out what the periodic trends are, we have to graph it and study them carefully.
Density: After graphing, you should be able to find that density usually increases along with the atomic number.
Melting and Boiling Point: After graphing, you should be able to find out that the melting and boiling points for metals usually increase from bottom to top of a group, and that for non-metals, they have an increase for melting and boiling points.
Ionization Energy: After graphing, you should be able to find out that it increases in a period (left to right), and it decreases as a group (top to bottom).Electronegativity: After graphing, you should be able to find that it increases from left to right.
Atomic Radius: After graphing, you should be able to find that it decreases as a period and increases as a group.
Tuesday, April 26, 2011
How many valence electrons?
Valence electrons:
1) Exist on the outermost shell of an atom
 2) Take part in chemical reactions
2) Take part in chemical reactions
3) Are all electrons except those IN THE CORE, THOSE IN FILLED D- or F- SUBSHELLS
Here is some important terminology:
An open shell is a shell that has fewer than the maximum amount of electrons occupying it. In this picture, the 2nd or outermost shell of the oxygen atom is the open shell. While it can hold 8 electrons, only 6 occupy the shell.
A closed shell is a shell that contains the maximum number of electrons that it can hold. In this example, the closed shell is the first one. It contains two electrons - the most that it can hold.
Let's look at the core notation for SULPHUR
S = 1s^22s^22p^63s^23p^4 CORE: [Ne]3s^23p^4
From the core notation, we look at the portion that is not within brackets. We then add up the superscripts of the s and p orbitals and get 6. Thus, we can conclude that there are six valence electrons in sulphur. We don't add up the superscripts of the d- or f- orbitals unless they aren't full.
Some unrelated terminology on the periodic table.
Periodic Law - the properties of certain chemical elements that occur every so often when the elements are placed in their order.
Ianthanides - the first row under the table that starts with lanthanum.
Actinides - the row under Ianthanides.
1) Exist on the outermost shell of an atom
 2) Take part in chemical reactions
2) Take part in chemical reactions3) Are all electrons except those IN THE CORE, THOSE IN FILLED D- or F- SUBSHELLS
Here is some important terminology:
An open shell is a shell that has fewer than the maximum amount of electrons occupying it. In this picture, the 2nd or outermost shell of the oxygen atom is the open shell. While it can hold 8 electrons, only 6 occupy the shell.
A closed shell is a shell that contains the maximum number of electrons that it can hold. In this example, the closed shell is the first one. It contains two electrons - the most that it can hold.
Let's look at the core notation for SULPHUR
S = 1s^22s^22p^63s^23p^4 CORE: [Ne]3s^23p^4
From the core notation, we look at the portion that is not within brackets. We then add up the superscripts of the s and p orbitals and get 6. Thus, we can conclude that there are six valence electrons in sulphur. We don't add up the superscripts of the d- or f- orbitals unless they aren't full.
Some unrelated terminology on the periodic table.
Periodic Law - the properties of certain chemical elements that occur every so often when the elements are placed in their order.
Ianthanides - the first row under the table that starts with lanthanum.
Actinides - the row under Ianthanides.
Thursday, April 21, 2011
Electronic Structure of the Atom!
What is the electronic structure of an atom? It's a notation that describes the orbitals in which electrons occupy and the total number of electrons in each orbit.
Remember back to when we learned about Neils Bohr? The guy in that picture? He proposed that electrons exist in specific energy states and when electrons absorb/emit specific amount of energy, it instantaneously moves from one orbital to the next.
Now for some vocab:
Energy level- amount of energy which electrons in atoms can possess ( "n"= number of energy levels)
Quantum of energy- energy difference between 2 particular energy levels
Ground state- when electrons of atoms are in their lowest possible energy level
Excited state- when 1 or more of an atom's electrons are in energy levels other than the lowest available level
Orbital- region of space occupied by an electron in particular energy level
Shell- set of all orbitals having the same "n" value
Subshell- set of orbitals of the same type
Orbitals are split into 4 different types: s, p, d, f
Each subshell consists of:
1: s-orbital
3: p-orbital
5: d-orbital
7: f-orbital
Pauli Exclusion Principle: maximum of 2 electrons can be placed in each orbit
This principle means that the maximum number of electrons in each subshell is:
2: s-subshell
6: p-subshell
10- d-subshell
14- f-subshell
This picture will help with writing the electronic configuration of atoms. For neutral atoms:
1) Always start with lowest energy level (Aufbau principle)
2) Figure out how many electron you have (neutral atom=atomic number)
3) Start at lowest energy level (1s) and keep adding on until none is left.
Each electron has an apposite spin designated by upward and downward arrows. An example is silicon. The last line of the picture shows the electronic configuration. Silicon has an atomic number of 14 thus it has 14 electrons at its neutral state. Notice that 2 electrons in 3p occupy separate orbitals and aren't paired? This is due to...
Hund's rule- when electrons occupy orbitals of equal energy, they don't pair up until they have to.
So in written form: 1s^22s^22p^63s^23p^2
The exponents represent the number of electrons.
Now for writing electronic configurations for ions.
For negative ions:
Add electrons (equal to charge) to last unfilled subshell starting where neutral atom left off. An example is N -3
Nitrogen in its neutral state has 7 electrons but with the charge of -3 it is actually 10
For positive ions:
1) Start with neutral atom and remove electrons from outermost shell depending on the charge.
2) If there are electrons in both s and p orbital of the outermost shell, elextrons in the p-orbital are removed first.
Core Notation- way of showing electron configuration in terms of core and outer electrons
A set of electrons belonging to a given atom can be divided into 2 subsets:
1) Core- set of electrons with configuration of the nearest noble gas (He, Ne, Ar, Kr, etc.) having an atomic number less than that of the atom being considered
2) Outer- consists of all electrons outside the core. Since core electrons, normally don't take part in chemical reactions.
So to find the core notation:
1) Locate the atom and note the noble gas at the end of the row and above the element
2) Write the electron configuration normally. BUT replace the part of the electron configuration corresponding to the configuration of the noble gas.
3) Write the noble gas in square brackets. Ex. [Ne]...
4) Follow the core symbol with the electron configuration of the remaining outer electrons.
Ex. Al: 1s^22s^22p^6 3s^23p^1
Ne: 1s^22s^22p^6 Bolded is the core. The rest is the outer. Therefore the core notation is: [Ne] 3s^23p^1
Exceptions!
Instead of: Cr --> [Ar] 4s^23d^4 (One electron short of half-filled subshell)
Cu--> [Ar] 4s^23d^9 (One electron short of a filled subshell)
In actuality:
Cr--> [Ar] 4s^13d^5 (Now both subshells are exactly half-filled)
Cu--> [Ar] 4s^13d^10 (4s^1 is exactly half-filled and 3d^10 is filled)
These 2 atoms indicate: A filled or exactly half-filled d-subshell is especially stable.
Link to awesome quiz to test your genius knowledge. :D
Remember back to when we learned about Neils Bohr? The guy in that picture? He proposed that electrons exist in specific energy states and when electrons absorb/emit specific amount of energy, it instantaneously moves from one orbital to the next.
Now for some vocab:
Energy level- amount of energy which electrons in atoms can possess ( "n"= number of energy levels)
Quantum of energy- energy difference between 2 particular energy levels
Ground state- when electrons of atoms are in their lowest possible energy level
Excited state- when 1 or more of an atom's electrons are in energy levels other than the lowest available level
Orbital- region of space occupied by an electron in particular energy level
Shell- set of all orbitals having the same "n" value
Subshell- set of orbitals of the same type
Orbitals are split into 4 different types: s, p, d, f
Each subshell consists of:
1: s-orbital
3: p-orbital
5: d-orbital
7: f-orbital
Pauli Exclusion Principle: maximum of 2 electrons can be placed in each orbit
This principle means that the maximum number of electrons in each subshell is:
2: s-subshell
6: p-subshell
10- d-subshell
14- f-subshell
This picture will help with writing the electronic configuration of atoms. For neutral atoms:
1) Always start with lowest energy level (Aufbau principle)
2) Figure out how many electron you have (neutral atom=atomic number)
3) Start at lowest energy level (1s) and keep adding on until none is left.
Each electron has an apposite spin designated by upward and downward arrows. An example is silicon. The last line of the picture shows the electronic configuration. Silicon has an atomic number of 14 thus it has 14 electrons at its neutral state. Notice that 2 electrons in 3p occupy separate orbitals and aren't paired? This is due to...
Hund's rule- when electrons occupy orbitals of equal energy, they don't pair up until they have to.
So in written form: 1s^22s^22p^63s^23p^2
The exponents represent the number of electrons.
Now for writing electronic configurations for ions.
For negative ions:
Add electrons (equal to charge) to last unfilled subshell starting where neutral atom left off. An example is N -3
Nitrogen in its neutral state has 7 electrons but with the charge of -3 it is actually 10
For positive ions:
1) Start with neutral atom and remove electrons from outermost shell depending on the charge.
2) If there are electrons in both s and p orbital of the outermost shell, elextrons in the p-orbital are removed first.
Core Notation- way of showing electron configuration in terms of core and outer electrons
A set of electrons belonging to a given atom can be divided into 2 subsets:
1) Core- set of electrons with configuration of the nearest noble gas (He, Ne, Ar, Kr, etc.) having an atomic number less than that of the atom being considered
2) Outer- consists of all electrons outside the core. Since core electrons, normally don't take part in chemical reactions.
So to find the core notation:
1) Locate the atom and note the noble gas at the end of the row and above the element
2) Write the electron configuration normally. BUT replace the part of the electron configuration corresponding to the configuration of the noble gas.
3) Write the noble gas in square brackets. Ex. [Ne]...
4) Follow the core symbol with the electron configuration of the remaining outer electrons.
Ex. Al: 1s^22s^22p^6 3s^23p^1
Ne: 1s^22s^22p^6 Bolded is the core. The rest is the outer. Therefore the core notation is: [Ne] 3s^23p^1
Exceptions!
Instead of: Cr --> [Ar] 4s^23d^4 (One electron short of half-filled subshell)
Cu--> [Ar] 4s^23d^9 (One electron short of a filled subshell)
In actuality:
Cr--> [Ar] 4s^13d^5 (Now both subshells are exactly half-filled)
Cu--> [Ar] 4s^13d^10 (4s^1 is exactly half-filled and 3d^10 is filled)
These 2 atoms indicate: A filled or exactly half-filled d-subshell is especially stable.
Link to awesome quiz to test your genius knowledge. :D
HAHAHA. SO FUNNY.
Tuesday, April 19, 2011
Modern Atoms
Modern atoms consist os electrons, protons and neutrons.
Remember the characteristics of each atom?
Proton , , mass = 1, charge = +1, located in the nucleus
Neutron, ,mass is >1, charge = 0, located in the nucleus
Electron, , does no = to 0, charge = -1, located around the nucleus
The # of protons = # of electrons
Atomic number (Z): number of protons found in a nucleus
- the atomic number = the number of protons = the number of electrons
Ions: atoms able to gain or lose electrons
- # of protons - # of electrons = the charge
Electrically charged atoms:
- negative charged ion are electrons added to a neutral atom
- positive charged ion are electrons removed from a neutral atom
Mass number: the total number of protons and neutrons
- atomic mass = # of protons + # of neutrons
- number of neutrons = mass # - atomic #
- mass # = # of protons + # of neutrons
Atomic mass: the average of an isotope
Isotopes: same number of protons and electrons but different number of neutrons
Natural Mixtures of Isotopes:
We have an element with 4 naturally occuring isotopes. Here are their atomic masses and percent abundance: a = 46 (25%), b = 47 (50%), c = 48 (15%), and d = 49 (10%). Find the atomic mass!
You try some with the link below!
http://www.chem4kids.com/activities.html


Remember the characteristics of each atom?
Proton , , mass = 1, charge = +1, located in the nucleus
Neutron, ,mass is >1, charge = 0, located in the nucleus
Electron, , does no = to 0, charge = -1, located around the nucleus
The # of protons = # of electrons
Atomic number (Z): number of protons found in a nucleus
- the atomic number = the number of protons = the number of electrons
Ions: atoms able to gain or lose electrons
- # of protons - # of electrons = the charge
Electrically charged atoms:
- negative charged ion are electrons added to a neutral atom
- positive charged ion are electrons removed from a neutral atom
Mass number: the total number of protons and neutrons
- atomic mass = # of protons + # of neutrons
- number of neutrons = mass # - atomic #
- mass # = # of protons + # of neutrons
Atomic mass: the average of an isotope
Isotopes: same number of protons and electrons but different number of neutrons
Natural Mixtures of Isotopes:
We have an element with 4 naturally occuring isotopes. Here are their atomic masses and percent abundance: a = 46 (25%), b = 47 (50%), c = 48 (15%), and d = 49 (10%). Find the atomic mass!
46 x 25% = 11.5
47 x 50% = 23.5
48 x 15% = 7.2
49 x 10% = 4.9
11.5 + 23.5 + 7.2 + 4.9 = 47.1 atomic mass units
You try some with the link below!
http://www.chem4kids.com/activities.html


Monday, April 18, 2011
Atomic Theory.
Aristotle: -believed that atoms were made from the 4 elements; earth, air, fire, and water Democritus: -in 300B.C, he believed that atoms were indivisible particles
Lavoisier (late 1700's) -stated earliest version of both the Law of Conservation and the Law of definite proportions
Proust (1799) - Proust believed that if a compound were to be broken down, it would still contain the same ratios as in the compound
Dalton (early 1800's) - believed that atoms are solid
-all atoms of given element are identical
-atoms of one element can combine with another to form chemical compounds
J.J. Thompson (1850) - proved existance of electrons
Rutherford (1905) - showed atoms had dense centres and electrons outside of them
-suggested atoms are mostly empty space
Niels Bohr (1885-1962) - studied gaseous samples of atoms
-proposed that electrons surrounds the nucleus in shells
The modern atom - an atom is the smallest particle of an element
-all atoms are made of three kinds of subatomic particles
-electrons
-protons
-neutrons

Monday, April 4, 2011
Percent Yield and Percent Purity!
Percent Yield= actual mass produced (grams) x 100
theoretical mass produced (grams)
The percent yield is found by dividing the actual mass of product formed by the mass of the product expected (this is found with stoichiometry).
Because not all the products will be used, the actual mass of product formed is always less than what was first expected.
Because not all the products will be used, the actual mass of product formed is always less than what was first expected.
E.g. If 12.5g of copper are reacted with an excess of chlorine, then 25.4g of copper(II)chloride are obtained. Calculate the percent yield.
First, balance the equation:
So we know that IN REALITY, 25.4g of CuCl2 were produced, this is our actual mass produced.
Only the pure part of the sample will react. So, before we can calculate how much product will form, we first need to know how much of the reactant is pure and available to react.
If a 5.67g sample of metal ore contains 4.65g of Nickel, what is the percent purity?
1Cu + 1Cl2 > 1CuCl2
We can use the 12.5g of copper to calculate the theoretical mass produced.
12.5g Cu x 1 mol Cu/63.5g x 1mol CuCl2/1 mol Cu x 134.5g/1 mol CuCl2 = 26.48g
So we know that IN REALITY, 25.4g of CuCl2 were produced, this is our actual mass produced.
Percent Yield= 25.4 x 100
26.5
So our percent yield is 95.9%.
Percent Purity= Mass of pure substance x 100
Mass of impure sample
Only the pure part of the sample will react. So, before we can calculate how much product will form, we first need to know how much of the reactant is pure and available to react.
If a 5.67g sample of metal ore contains 4.65g of Nickel, what is the percent purity?
Percent Purity= 4.65 x 100
5.67
GOOD DAY!
Subscribe to:
Comments (Atom)