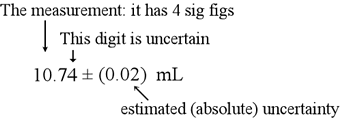Basis for separation: different components , different properties
Strategy: Divide a process that decreases between different properties and different components.
Separation:
- The more similar properties are it is difficult to separate them
ex. oil and water
Other Techniques of Separation:
Filtration: select components by the size of their particles
Floatation: select components by density
Crystallization and Extraction: select components by solutions
Distillation: select components by their boiling points
Hand Separation and Evaporation:
- Hand separation (solid and solid)
- Evaporation (solid dissolves in liquid)
- Boiling the liquid the solid remains as it is
Filtration:
- solids no dissolve in liquids
Crystallization:
- Solid and Liquid
- Solids are separated by filtration
- Solution is a solid
- Solid is cooled and creates pure crystals
- Crystals are then filtered again
Gravity Separation:
- solid based
- separation allowing denser components to settle
Solven Extraction:
- better if a solvent mixture dissolve in only component.
- MECHANICAL MIXTURES use liquids to dissolve solid
- Solution: the solvent will dissolve substances and will leave unwanted substances behind.
Distillation:
- Liquid to Liquid
- Low boiling points can cause a mixture to vaporize
- The liquid with the lowest boiling temperature will boil first
Chromatography:
- Different speed components can flow over the material at different speed.
- Are able to separate very complex mixtures (drugs, plastic, foods etc.
Sheet Chromatography:
1) Paper Chromatography:
- Is in a stationary phase
- appear as spots separated on a paper after it dries
2) Thin Layer Chromatography:
- In a stationary phase that absorbs (AL2, O3, SiO2) [covering it with glass will absorb more quickly]
- appear as spots on a sheet
 First of all, we heated the crucible with a bunsen burner for a couple of minutes to make sure it was dry.
First of all, we heated the crucible with a bunsen burner for a couple of minutes to make sure it was dry. 




















 Density of water is 1000 g/L or 1.0 g/mL
Density of water is 1000 g/L or 1.0 g/mL