Today, we reviewed how to write the chemical formulas of compounds and how to name them as well.
Ionic compounds are compounds made up of at least two particles that form bonds by giving or receiving electrons. Ionic compounds are often made up of metals (which give up an electron) and non-metals (which do the receiving).
E.g.
Calcium gives up its two valence electrons (now it is stable) to two chlorine atoms (now they are stable as well). The charges balance out and calcium chloride is formed.
NOTE: Multivalent metals (metals that can form ions in more than one way) can often have names ending in -ic (for the higher charge) and -ous (for the lower charge)
For example: Cupric is Copper (II) and Cuprous is Copper (I)
When naming these compounds, make sure that the metal is mentioned first and that the suffix of the non-metal is changed to -ide.
Covalent Compounds share electrons. This bond occurs between non-metal elements.
Eg.
The hydrogen atoms share electrons with the oxygen atom creating a water molecule.
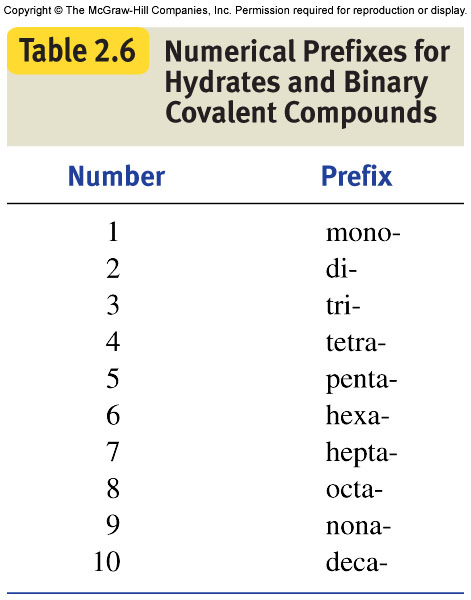
When naming, take into account these prefixes:
Here is a link to some practice worksheets:
http://misterguch.brinkster.net/pra_namingwkshts.html
GOOD LUCK!
No comments:
Post a Comment