-STP, otherwise known as Standard Tempereature and Pressure, is a standard conditon to compare volume of gases
-STP is equal to.. 1 atmosphere of pressure and with a temperature of 0°C or 273.15 K (Kelvins)
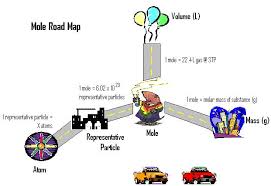
-at STP, 1 mole of gas occupies 22.4 L
THEREFORE, new conversion factors : P
22.4 L of gas OR 1 mole of gas
1 mole of gas 22.4 L of gas
Basically, you can add these two new conversions to the mole conversions map from before. You can only use the two conversions between mole and gas.
Ex. A cylinder contains 15.85 moles of N2 gas at STP. What is the volume of the gas?
1 mole
No comments:
Post a Comment