SO. Today, we had our first laboratory experiment! The lab experiment title was chemical and physical change Basically, we received 4 different chemicals, A, B, C and D. From these 4 chemicals, we made 6 new chemicals from the mixtures of: AB, AC, AD, BC, BD, and CD. We conducted these mixtures on a glass square and recorded what happened when each of them were mixed together. After we were done the experiment, we carefully washed and dried the glass square, all the test tubes and eye droppers and put them back along with the test tube rack. We washed our hands and spent the rest of the class working on our lab reports, which are due friday.
Wednesday, September 29, 2010
Monday, September 27, 2010
It's... MATTER!
 So what is MATTER?
So what is MATTER? Matter is ANYTHING that has mass or takes up space!
The two types of matter: pure substances and mixtures.
Pure substances have one set of properties and one kind of particle like elements (made up of metal, non metal, and metalloid atoms) and compounds (ionic or covalently combined atoms)
Mixtures are made up of more than one set of properties or substances. They can either be homogeneous (they are uniform and appear as though they are one component e.g. the glass of milk) or heterogeneous (don't appear uniform, as though they have more than one component e.g. the salad dressing)
CHANGES TO MATTER!
Matter can change in two ways - physically or chemically.
You can tell that a change is physical when no new substances have been formed and the change that has resulted can be reversed.
You can tell that a change is chemical when new substances are produced and the result is irreversible.
Here's a great song to teach you about physical and chemical changes!
 Some more facts about matter!
Some more facts about matter!-Matter cannot be created or destroyed
-There are three states of matter - solid (rigid, doesn't change shape easily), liquid (takes the shape of the container), and gas (moves freely)
PLASMA is often considered and fourth state of matter. It is similar to gas but flows like a liquid. Plasma is something you would find in neon signs like this one:
Thursday, September 23, 2010
Unitary Rate To Doom...is in 4 days, 96 hours, 5760 minutes, and 345600 seconds.
The quiz on unitary rate conversion is on Monday, Sept. 27th!
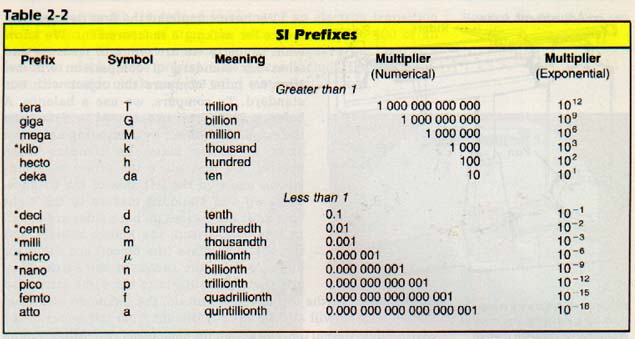 | ||
| Here's the SI prefixes and their values. |
Ready for the quiz? Try this one! http://chemistry.about.com/library/weekly/aa021903a.htm?lastQuestion=1&answers=0&submit=Next+Question+%3E%3E&ccount=1
Don't get it? Here's a video that might help you:
Note: Watch the video up until 1:40 and it should give you the basic idea of how to convert units. =)
Tuesday, September 21, 2010
Scientific Notation and Unitary Rates
Scientific notation: is used to express very large or very small numbers using powers of 10.
In scientific notation it's written like:
a × 10b
In scientific notation, the a can not be smaller then 0 or greater then 10 therefore we must move the decimal place.
Examples would be:
| Ordinary decimal notation | Scientific notation (normalized) |
|---|---|
| 300 | 3×102 |
| 4,000 | 4×103 |
| 5,720,000,000 | 5.72×109 |
| 0.0000000061 | 6.1×10−9 |
We can also express scientific notation in standard form...
Ex. a) 3.25 x 109
= 3 250 000 000
b) 8.76 x 10−3
= 0.00876
Instead of doing the calculations by hand we can plug it into a calculator by:
- Plugging in the number (example 3.25)
- Press EXP or EE
- Then an exponent looking like 00 will show up
- Plug in however many numbers you want in the exponent
- Press the "=" sign and you have your answer!
Unitary Rates:
Ex. If 1m is 100 cm, what is 1 m2?
1m2 = 10000 cm2
Practices:
Convert a) 8.1 m3 to km3
b) 2.4 m3 to 8.2 cm2
Subscribe to:
Comments (Atom)

